Scientists from Johns Hopkins University and the University of Bonn in Germany have applied advances in optogenetics to terminate arrhythmias in mice.
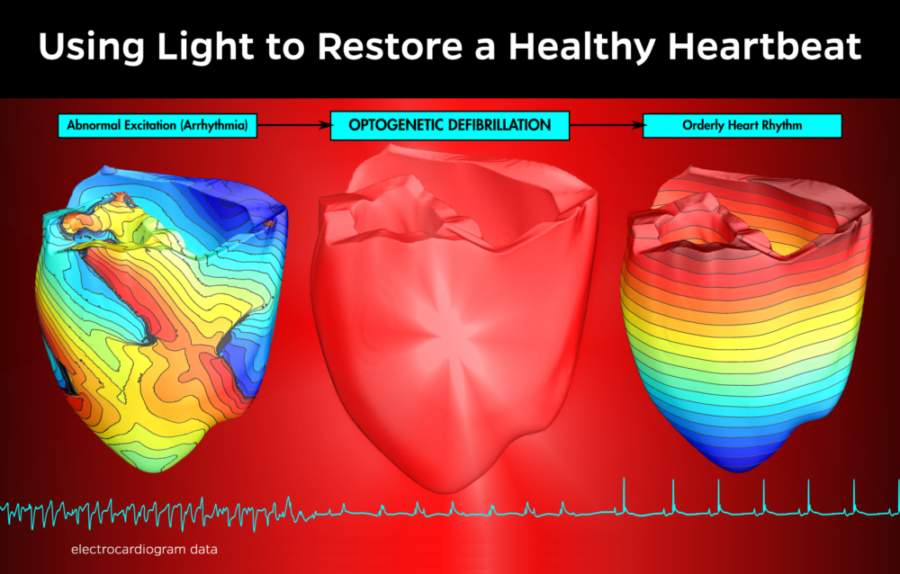
In a new study published online yesterday in the Journal of Clinical Investigation, authors prove the concept of optogenetic defibrillation, where epicardial illumination can effectively terminate ventricular tachycardia—a potential alternative to implantable defibrillators.
"Strong electrical shocks can damage the heart and cause severe pain," write the authors. "Our results... could potentially be translated into humans to achieve nondamaging and pain-free termination of ventricular arrhythmia."
Rather than the "abrupt punch" of an electrical defibrillation, optogenetic defibrillation resulted in a "gradual slowing of the arrhythmia which eventually led to termination," says Patrick Boyle, PhD of Johns Hopkins University in the video above.
How does it work? Optogenetics utilizes genetically-engineered proteins that react to light and create a an electric current in response. After these proteins are embedded in the heart, the heart can be defibrillated by a one second pulse of light.
To prove the concept for human patients, Johns Hopkins University researchers used a computer model to simulate how red light could penetrate human heart tissue and alter the electrical behavior of cells in the heart.
For patients with ventricular tachycardia, implantable optical defibrillators are still some time away from becoming a reality, however.
"It will still take at least five to ten years," says Phillip Sasse, MD, of the University of Bonn in the Johns Hopkins University press release. "The new method is still in the stage of basic research."
Even at the early stages, it's an exciting innovation on the new frontier of cardiac optogenetics.
