NSTEMI: Myocardial Revascularization
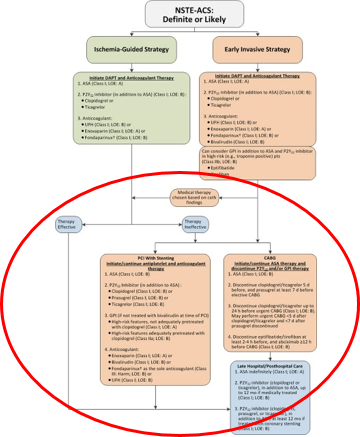
Algorithm for Management of Patients With Definite or Likely NSTE-ACS
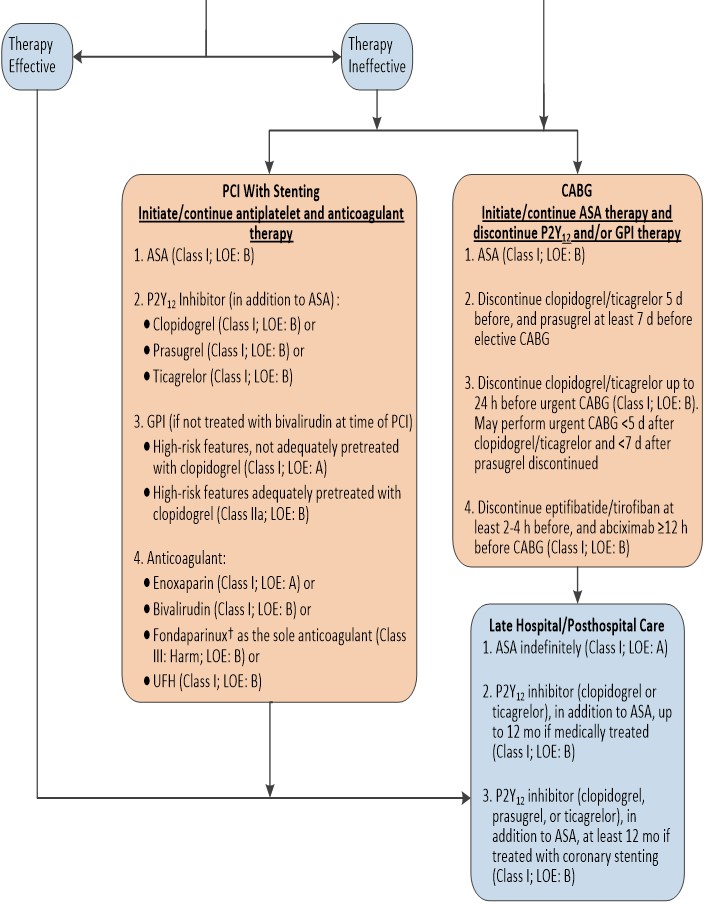
Percutaneous Coronary Intervention Considerations
| Recommendation | Class | Level |
| A strategy of multivessel PCI, in contrast to culprit lesion-only PCI, may be reasonable in patients undergoing coronary revascularization as part of treatment for NSTE-ACS. | IIb | B |
Antiplatelet and Anticoagulant Therapy: Oral and Antiplatelet Agents
| Recommendation | Class | Level |
| Patients already taking daily aspirin before PCI should take 81 mg to 325 mg non–enteric-coated aspirin before PCI. | I | B |
| Patients not on aspirin therapy should be given non–enteric-coated aspirin 325 mg as soon as possible before PCI. | I | B |
| After PCI, aspirin should be continued indefinitely at a dose of 81 mg to 325 mg daily. | I | B |
| A loading dose of a P2Y12 receptor inhibitor should be given before the procedure in patients undergoing PCI with stenting. Options include: • Clopidogrel: 600 mg or • Prasugrel#: 60 mg or • Ticagrelor║: 180 mg | I | B |
| In patients with NSTE-ACS and high-risk features (e.g., elevated troponin) not adequately pretreated with clopidogrel or ticagrelor, it is useful to administer a GP IIb/IIIa inhibitor (abciximab, double-bolus eptifibatide, or high-dose bolus tirofiban) at the time of PCI. | I | A |
| In patients receiving a stent (bare-metal stent or drug-eluting stent [DES]) during PCI for NSTE-ACS, P2Y12 inhibitor therapy should be given for at least 12 months. Options include • Clopidogrel: 75 mg daily or • Prasugrel#: 10 mg daily or • Ticagrelor║: 90 mg twice daily | I | B |
| It is reasonable to choose ticagrelor over clopidogrel for P2Y12 inhibition treatment in patients with NSTE-ACS treated with an early invasive strategy and/or coronary stenting. | IIa | B |
| It is reasonable to choose prasugrel over clopidogrel for P2Y12 treatment in patients with NSTE-ACS who undergo PCI who are not at high risk of bleeding complications. | IIa | B |
| In patients with NSTE-ACS and high-risk features (e.g., elevated troponin) treated with UFH and adequately pretreated with clopidogrel, it is reasonable to administer a GP IIb/IIIa inhibitor (abciximab, double-bolus eptifibatide, or high-bolus dose tirofiban) at the time of PCI. | IIa | B |
| After PCI, it is reasonable to use 81 mg per day of aspirin in preference to higher maintenance doses. | IIa | B |
| If the risk of morbidity from bleeding outweighs the anticipated benefit of a recommended duration of P2Y12 inhibitor therapy after stent implantation, earlier discontinuation (e.g., <12 months) of P2Y12 inhibitor therapy is reasonable. | IIa | C |
| Continuation of DAPT beyond 12 months may be considered in patients undergoing stent implantation. | IIb | C |
| Prasugrel should not be administered to patients with a prior history of stroke or transient ischemic attack. | III | B |
Antiplatelet and Anticoagulant Therapy: GP IIb/IIIa Inhibitors
| Recommendation | Class | Level |
| In patients with NSTE-ACS and high-risk features (e.g., elevated troponin) and not adequately pretreated with clopidogrel or ticagrelor, it is useful to administer a GP IIb/IIIa inhibitor (abciximab, double-bolus eptifibatide, or high-dose bolus tirofiban) at the time of PCI. | I | A |
| In patients with NSTE-ACS and high-risk features (e.g., elevated troponin) treated with UFH and adequately pretreated with clopidogrel, it is reasonable to administer a GP IIb/IIIa inhibitor (abciximab, double-bolus eptifibatide, or high-dose bolus tirofiban) at the time of PCI. | IIa | B |
Anticoagulant Therapy in Patients Undergoing PCI
| Recommendation | Class | Level |
| An anticoagulant should be administered to patients with NSTE-ACS undergoing PCI to reduce the risk of intracoronary and catheter thrombus formation. | I | C |
| Intravenous UFH is useful in patients with NSTE-ACS undergoing PCI. | I | C |
| Bivalirudin is useful as an anticoagulant with or without prior treatment with UFH in patients with NSTE-ACS undergoing PCI. | I | B |
| An additional dose of 0.3 mg/kg IV enoxaparin should be administered at the time of PCI to patients with NSTE-ACS who have received fewer than 2 therapeutic subcutaneous doses (e.g., 1 mg/kg SC) or received the last subcutaneous enoxaparin dose 8 to 12 hours before PCI. | I | B |
| If PCI is performed while the patient is on fondaparinux, an additional 85 IU/kg of UFH should be given intravenously immediately before PCI because of the risk of catheter thrombosis (60 IU/kg IV if a GP IIb/IIIa inhibitor used with UFH dosing based on the target-activated clotting time). | I | B |
| In patients with NSTE-ACS, anticoagulant therapy should be discontinued after PCI unless there is a compelling reason to continue such therapy. | I | C |
| In patients with NSTE-ACS undergoing PCI who are at high risk of bleeding, it is reasonable to use bivalirudin monotherapy in preference to the combination of UFH and a GP IIb/IIIa receptor antagonist. | IIa | B |
| Performance of PCI with enoxaparin may be reasonable in patients treated with upstream subcutaneous enoxaparin for NSTE-ACS. | IIb | B |
| Fondaparinux should not be used as the sole anticoagulant to support PCI in patients with NSTE-ACS due to an increased risk of catheter thrombosis. | III | B |
Dosing of Parenteral Anticoagulants During PCI
| Drug | In Patients Who Have Received Prior Anticoagulant Therapy | In Patients Who Have Not Received Prior Anticoagulant Therapy |
| Enoxaparin | ·For prior treatment with enoxaparin, if last SC dose was administered 8-12 h earlier or if <2 therapeutic SC doses of enoxaparin have been administered, an IV dose of enoxaparin 0.3 mg/kg should be given ·If the last SC dose was administered within prior 8 h, no additional enoxaparin should be given | ·0.5 mg/kg–0.75 mg/kg IV loading dose |
| Bivalirudin | ·For patients who have received UFH, wait 30 min, then give 0.75 mg/kg IV loading dose, then 1.75 mg/kg/h IV infusion ·For patients already receiving bivalirudin infusion, give additional loading dose 0.5 mg/kg and increase infusion to 1.75 mg/kg/h during PCI | ·0.75 mg/kg loading dose, 1.75 mg/kg/h IV infusion |
| Fondaparinux | ·For prior treatment with fondaparinux, administer additional IV treatment with anticoagulant possessing anti-IIa activity, considering whether GPI receptor antagonists have been administered | N/A |
| UFH | ·IV GPI planned: additional UFH as needed (e.g., 2,000–5,000 U) to achieve ACT of 200–250 s ·No IV GPI planned: additional UFH as needed (e.g., 2,000–5,000 U) to achieve ACT of 250–300 s for HemoTec, 300–350 s for Hemochron | ·IV GPI planned: 50–70 U/kg loading dose to achieve ACT of 200–250 s ·No IV GPI planned: 70–100 U/kg loading dose to achieve target ACT of 250–300 s for HemoTec, 300–350 s for Hemochron |
Timing of Urgent CABG in Patients With NSTE-ACS in Relation to Use of Antiplatelet Agents
| Recommendation | Class | Level |
| Non–enteric-coated aspirin (81 mg to 325 mg daily) should be administered preoperatively to patients undergoing CABG. | I | B |
| In patients referred for elective CABG, clopidogrel and ticagrelor should be discontinued for at least 5 days before surgery and prasugrel for at least 7 days before surgery. | I | B |
| In patients referred for urgent CABG, clopidogrel and ticagrelor should be discontinued for at least 24 hours to reduce major bleeding. | I | B |
| In patients referred for CABG, short-acting intravenous GP IIb/IIIa inhibitors (eptifibatide or tirofiban) should be discontinued for at least 2 to 4 hours before surgery and abciximab for at least 12 hours before to limit blood loss and transfusion. | I | B |
| In patients referred for urgent CABG, it may be reasonable to perform surgery less than 5 days after clopidogrel or ticagrelor has been discontinued and less than 7 days after prasugrel has been discontinued. | IIb | C |