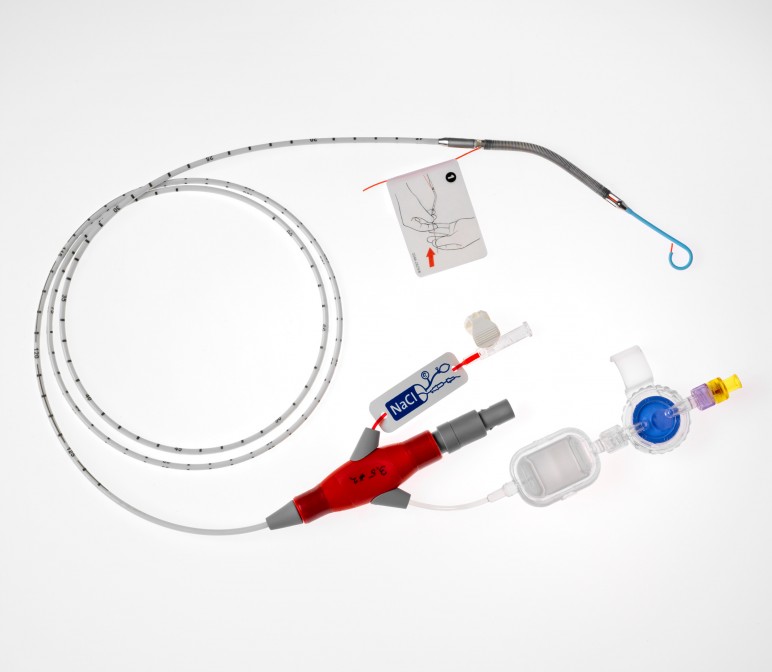
Pictured: Impella CP.
Cardiovascular technology has been all over financial news recently as Abiomed's Impella CP roll-out continued to be strong last quarter and their Impella RP (Right Percutaneous) system received FDA approval under a Humanitarian Device Exemption.
The Impella devices, tiny heart pumps introduced via catheter by the femoral artery, allow cardiovascular professionals to support the heart in a minimally invasive way, avoiding open heart surgery in many cases. The devices mark a significant advancement in cardiovascular treatment for patients with extreme surgical risk.
Continue reading Impella RP Approved, Impella CP Devices Continue Roll-Out