Yesterday, the Food and Drug Administration approved Abbott's Absorb stent, the first fully dissolving stent to be approved by the FDA.
A 10-person FDA advisory panel, including Dr. George Vetrovec of Virginia Commonwealth University, voted unanimously in March to affirm that the stent's benefits and efficacy outweigh its risks. The same panel voted 9-1 in favor of its safety profile, despite the risk of blood clots for some patients.
"This is presumably a better technology going forward - at least that's the theory - but it will take years to prove," said Vetrovec to the Associated Press.
FDA approves Absorb with advisory panel warning
The Absorb stent was evaluated and approved under current FDA regulatory guidance for the approval of coronary drug-eluting stents, requiring research to provide evidence for "non-inferiority" to an FDA-approved drug-eluting stent and a primary outcome measure evaluating safety and effectiveness at one year.
The ABSORB III trial was conducted and published in October, 2015 to meet this guidance, showing a "clinically comparable" rate of major cardiac adverse events of 7.8 percent compared to Abbott's FDA-approved metal drug-coated stent, Xience, which had a rate of 6.1 percent.
The trial also showed 0.8 percent higher rates of stent thrombosis - where a blood clot forms on the surface of the stent - compared to Xience, though the FDA advisory panel noted that "there was a strong dependence on reference vessel diameter."
"The current generation of Absorb has thicker struts than Xience, and this it is biologically plausible that in very small vessels, the space occupying effect of larger struts might negatively impact outcomes," reads the Sponsor Executive Summary of the FDA Advisory Committee Meeting on March 15.
The result? A strictly-worded warning: "If quantitative imaging determines a vessel size < 2.5 mm, do not implant Absorb. Implantation of the device in vessels < 2.5 mm may lead to an increased risk of adverse events such as myocardial infarction and scaffold thrombosis."
Absorb outcomes might shine beyond one year
What's exciting about the bioresorbable stent, however, is its potential for better outcomes beyond one year.
Absorb is designed to dissolve completely after 2-3 years, theoretically allowing stented vessels to return to normal. "Without a permanent metallic implant left behind, a vessel treated with the Absorb stent has the ability to move, flex, and pulsate naturally," says an informational video from Abbott Vascular.
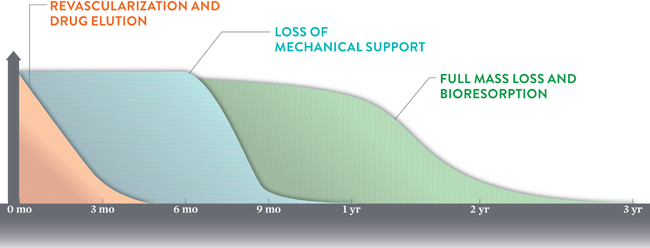
"We have good theoretical reasons to believe that by getting rid of the stent, and allowing the coronary artery to restore its normal shape, that will prevent many of those late events," said Dr. Gregg Stone, principal investigator on the ABSORB III trial, to the Associated Press.
Abbot plans "careful launch" of Absorb
The Wall Street Journal reported today that Abbott "plans a careful launch of the stent."
"We're going to roll this out commercially in a phased way, starting with the sites that have had experience with our clinical trials and proceeding from there," said Charles Simonton, chief medical officer of Abbott Vascular, to the WSJ.
Are you excited or concerned about this new tech? Leave a comment!
