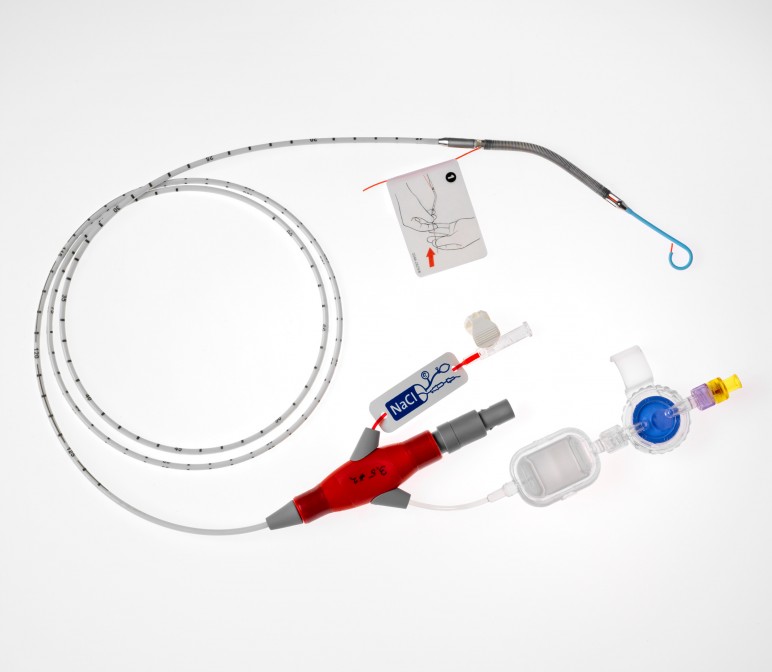
Pictured: Impella CP.
Cardiovascular technology has been all over financial news recently as Abiomed's Impella CP roll-out continued to be strong last quarter and their Impella RP (Right Percutaneous) system received FDA approval under a Humanitarian Device Exemption.
The Impella devices, tiny heart pumps introduced via catheter by the femoral artery, allow cardiovascular professionals to support the heart in a minimally invasive way, avoiding open heart surgery in many cases. The devices mark a significant advancement in cardiovascular treatment for patients with extreme surgical risk.
The Impella devices currently on the market are Left-Ventricular Assist Devices (LVADs), but the new Impella RP that received approval is the first heart pump approved to offer support for right-sided heart failure.
Abiomed's recently launched Impella CP device features a significant improvement of blood flow over previous models—4 liters of blood per minute versus 2.5 liters per minute—which has driven spread of Impella devices.
Abiomed reports that 931 hospitals are now using Impella devices, 583 of which are using the improved Impella CP device. Is your hospital using Impella? Are you planning on it?
Learn More about Impella
- ACVP's Cardio Conference in Harrisonburg, VA on March 14 will feature Lesley Lauritsen presenting "a practical guide" to Impella use. Earn your CEUs and learn more about the fascinating new tech. Register today!
- We also found some excellent resources on Abiomed's website. Check out their Impella Quick Skills Videos.